ONTOZRY®▼ (cenobamate) Efficacy

This promotional website is managed and funded by Angelini Pharma and is intended for UK and Ireland healthcare professionals only
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events and product complaint should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard for the UK or www.hpra.ie for Ireland. Adverse events and product complaint should also be reported to Angelini Pharma on (UK) +44 2034889643, (IRE) +353 1 584 4671 or UKIReporting@angelinipharma.com
The efficacy of ONTOZRY® (cenobamate) in clinical trials
The efficacy of ONTOZRY® for the treatment of uncontrolled focal onset epilepsy was evaluated in two multicentre, randomised, double-blind, placebo-controlled efficacy and safety studies and in one open-label safety study.1–3
ONTOZRY® (cenobamate) C017 pivotal study: seizure frequency and seizure freedom
C017 was a Phase IIb, multicentre, randomised, double-blind, placebo-controlled, dose-response study with an optional open-label extension (OLE).1
Reducing seizure frequency with ONTOZRY® (cenobamate): primary endpoints
The two primary endpoints of C017 were:1
- % of patients achieving ≥50% reduction from baseline in focal seizure frequency (during the 12-week maintenance phase of the double-blind period) when adding placebo or ONTOZRY® to an ongoing regimen of 1-3 anti-seizure medications (ASMs) (Standard of care; SoC)*.
- % change from baseline in focal seizure frequency averaged over 28 days in the 18-week double blind treatment period.
Adjunctive ONTOZRY® reduces the frequency of drug-resistant focal onset seizures by ≥50% in over half of patients.1

*SoC was treatment with up to three concomitant anti-seizure medications.1
ONTOZRY® (cenobamate) and seizure freedom: secondary endpoints
The key secondary endpoints of C017 were:1
- % of patients with ≥75%, ≥90% and 100% reduction in seizure frequency (measured over a 12-week maintenance phase) when adding placebo or ONTOZRY® to SoC.
- % change from baseline in seizure frequency per 28 days by seizure type (measured over a 12-week maintenance phase) when placebo or ONTOZRY® was added to SoC.
Seizure freedom is possible with adjunctive ONTOZRY® for some drug-resistant patients†1

Adjunctive ONTOZRY® is associated with dose-dependent improvements in ≥75%, ≥90% and 100% responder rates.1
Adjunctive ONTOZRY® reduces seizure frequency in patients with epilepsy with different focal seizure subtypes.1

†When added to standard of care (an ongoing regimen of 1-3 ASMs).

Sustained seizure freedom: results from C017 OLE study post hoc analysis
Reductions in seizure freedom were maintained with adjunctive ONTOZRY® at >3 and up to 4 years when added to an ongoing regimen of 1-3 ASMs.4

During the 4 years of the OLE, 11.9% of patients achieved ≥2 consecutive years of seizure freedom with adjunctive ONTOZRY®.4

Around 62% of patients continue with ONTOZRY® for at least 4 years.4

Around 57% of patients are estimated to continue with adjunctive ONTOZRY® for at least 8 years.5


ONTOZRY® (cenobamate) Phase IIb clinical trial: C017 study design
C017 was a Phase IIb, multicentre, randomised, double-blind, placebo-controlled efficacy and safety study with an optional OLE.1
The study enrolled participants who had uncontrolled focal-onset seizures despite a history of treatment with 1-3 concomitant ASMs at stable doses for at least 4 weeks prior to screening. Prior to enrolment, had received a median of 3 prior ASMs (range 2–4). These might or might not have been ongoing at the time of the trial, with 74% (n=322/437) of patients taking 2-3 concomitant ASMs during the study.1
The patient inclusion criteria included:1
- Aged 18-70 years
- Focal epilepsy, uncontrolled despite treatment with at least one ASM within the last 2 years, as defined by the international league against epilepsy (ILAE).
- Taking 1-3 concomitant ASMs at stable doses for at least 4 weeks before screening.
- NOT having taken diazepam, phenytoin, or phenobarbital within 1 month of screening because of the potential for drug-drug interactions with ONTOZRY®.
The baseline demographic and epilepsy related characteristics were generally similar across groups:1

Participants (n=437) were randomised 1:1:1:1 to receive either placebo or ONTOZRY® 100 mg/day, 200 mg/day or 400 mg/day. Dosing was titrated over 6 weeks, as shown in the diagram below.1
In the 400 mg/day arm, once the 200 mg/day dose was reached, the daily dose was up-titrated by 100 mg at weekly intervals to the target dose. If a patient did not tolerate the next highest dose, the dose could be reduced to the previous dose.1

In the 8-week baseline period, patients were evaluated for seizure frequency and type so that this data could be compared to the 12-week maintenance phase in order to calculate the change in focal seizure frequency.1
At completion of the double blind period, patients who continued to meet study eligibility, with the exception of the seizure frequency requirement, could enrol in an OLE whereby patients underwent a blinded 2-week conversion to a target dose of ONTOZRY® 300 mg/day.1
‡Initial starting dose of ONTOZRY® in the original, faster titration schedule was 100 mg/day, with weekly increments of 100 mg/day until the target dose was reached. The amended titration schedule reduced the initial starting dose to 50 mg/day and slowed the titration rate to improve tolerability.1
ONTOZRY® (cenobamate) C013 supportive study and reduced frequency of seizures
As a supportive study to C017, C013 was a multicentre, randomised, double- blind, placebo-controlled, parallel-group efficacy and safety study.2
ONTOZRY® (cenobamate) Phase IIb clinical trial: C017 study design
Primary endpoint: % change from baseline in focal seizure frequency per 28 days (measured over a 12-week double-blind treatment period) when placebo or ONTOZRY® was added to an ongoing regimen of 1-3 ASMs (SoC).2
Adjunctive ONTOZRY® significantly reduced the frequency of drug-resistant focal-onset seizures vs placebo.2

Secondary endpoint: % of patients with ≥50% reduction in seizure frequency (measured over a 12-week double-blind treatment period) when placebo or ONTOZRY® was added to an ongoing regimen of 1-3 ASMs (SoC).2
Adjunctive ONTOZRY® reduces the frequency of drug-resistant focal-onset seizures by ≥50% in over half of patients.2

ONTOZRY® (cenobamate) Phase 2 clinical trial: C013 study design
C013 was a Phase II, multicentre, randomised, double-blind, placebo-controlled parallel group efficacy and safety study.2
The patient inclusion criteria included:2
- Aged 18-65 years.
- Focal epilepsy, drug resistant as defined by the ILAE*
- Taking 1-3 concomitant ASMs at stable doses for at least 12 weeks before randomisation
- NOT having taken phenytoin or phenobarbital because of the potential for drug-drug interaction with ONTOZRY®
The baseline demographic and epilepsy-related characteristics were generally similar in each treatment group:2

Participants (n=222) were randomised 1:1 to receive either placebo or 200 mg/day ONTOZRY® which was titrated up over 6 weeks.2

Patients who continued to meet study eligibility, with the exception of seizure frequency requirement, could enrol in an OLE.6
*Defined as the failure of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.7
**The OLE target dose was initially 200 mg/day but was increased to 400 mg/day ~2 years after OLE initiation.6
ONTOZRY® (cenobamate) C021 safety study
C021, was a Phase III, open-label, multicentre, long-term, safety and pharmacokinetic study of ONTOZRY® as adjunctive therapy in patients with partial onset seizures.3
ONTOZRY® (cenobamate) C021 post hoc analysis: responder rates and seizure frequency
Aside from the findings of the study that are related to the safety profile of ONTOZRY®, the study data also demonstrated that patients with varied disease backgrounds responded to adjunctive ONTOZRY®.8
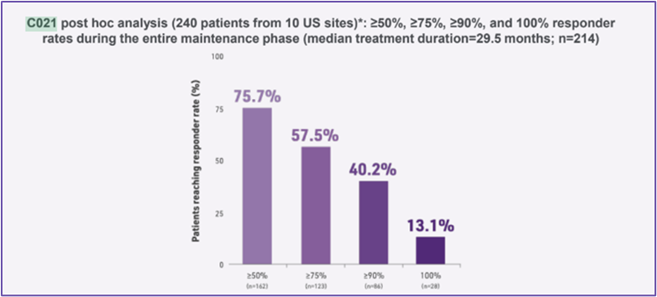
Post hoc analysis (240 patients from 10 US sites): ≥50%, ≥75%, ≥90%, and 100% responder rates during the entire treatment period, during the titration phase, and during the maintenance phase (median maintenance treatment duration=29.5 months; n=214).9
Responder rates observed in a C021 post hoc analysis support the efficacy observed with adjunctive ONTOZRY® in the C017 trial.9
In a post hoc analysis of 240 patients in the safety study C021, 36.3% (n=87) were seizure-free at any consecutive ≥12-month duration of the study.9
Post hoc analysis showed that the mean duration of 100% seizure reduction was 23.5 months and the median duration of exposure for the 240 patients in the post hoc open-label study was 30.2 months.9

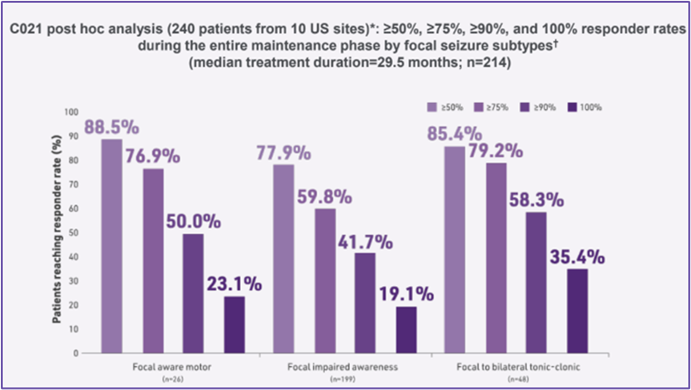
C021 post hoc analysis (240 patients from 10 US sites)*: ≥50%, ≥75%, ≥90%, and 100% responder rates during the entire maintenance phase by focal seizure subtypes (median maintenance treatment duration=29.5 months; n=214).9
Responder rates with adjunctive ONTOZRY® were consistent in most patients with different focal seizure subtypes.9
ONTOZRY® (cenobamate) Phase III clinical trial C021 study design
C021 was a Phase III, open-label, long-term safety study with a primary end point of long-term safety profile and tolerability of ONTOZRY®.3
The participant inclusion criteria for the study was the following:3
- Aged 18-70 years old
- Focal epilepsy, uncontrolled despite treatment with at least 1 ASM within the past 2 years, as defined by the ILAE
- Taking 1-3 concomitant ASMs at stable doses for at least 3 weeks before initiation of ONTOZRY®
- No history of any drug-induced rash or hypersensitivity reaction
The initial dose of ONTOZRY® was lower (12.5 mg/day) and the titration rate was slower (every 2 weeks for 12 weeks) than in previous clinical studies. Except for patients taking concomitant phenytoin or phenobarbital, concomitant ASMs could be removed, added, or adjusted. The ONTOZRY® dose could be adjusted during the titration phase, as clinically needed, including downward dose adjustments for tolerability during the maintenance phase for all patients once the target dose of 200 mg/day was reached.3

At data cut off, 1339 patients had received ≥1 treatment dose (safety population).3


Abbreviations
ASM, anti-seizure medication; EMA, european medicines agency; FDA, food and drug administration; ILAE, international league against epilepsy; OLE, open-label extension; SoC, standard of care.
- Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. The Lancet Neurology. 2020;19(1):38-48. doi:https://doi.org/10.1016/s1474-4422(19)30399-0
- Chung SS, French JA, Kowalski J, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311-e2322. doi:https://doi.org/10.1212/wnl.0000000000009530
- Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open‐label safety study. Epilepsia. 2020;61(6):1099-1108. doi:https://doi.org/10.1111/epi.16525
- Klein P, Aboumatar S, Brandt C, et al. Long-term Efficacy and Safety From an Open-Label Extension of Adjunctive Cenobamate in Patients With Uncontrolled Focal Seizures. Neurology. 2022;99(10):e989-e998. doi:https://doi.org/10.1212/WNL.0000000000200792
- Sander JW, Rosenfeld WE, Halford JJ, Steinhoff BJ, Biton V, Toledo M. Long‐term individual retention with cenobamate in adults with focal seizures: Pooled data from the clinical development program. Epilepsia. 2021;63(1):139-149. doi:https://doi.org/10.1111/epi.17134
- Rosenfeld WE, Abou‐Khalil B, Aboumatar S, et al. Post hoc analysis of a phase 3, multicenter, open‐label study of cenobamate for treatment of uncontrolled focal seizures: Effects of dose adjustments of concomitant antiseizure medications. Epilepsia. 2021;62(12):3016-3028. doi:https://doi.org/10.1111/epi.17092
- Sperling MR, Abou‐Khalil B, Aboumatar S, et al. Efficacy of cenobamate for uncontrolled focal seizures: Post hoc analysis of a Phase 3, multicenter, open‐label study. Epilepsia. 2021;62(12):3005-3015. doi:https://doi.org/10.1111/epi.17091

MAT-UKI-0283-P | January 2026
 HarmoniaMentis
HarmoniaMentis

