Introducing ONTOZRY®▼ (cenobamate)

Introducing ONTOZRY®▼ (cenobamate)
Great Britain: ONTOZRY®▼ is indicated for the adjunctive treatment of focal-onset seizures with or without secondary generalisation in adult patients with epilepsy who have not been adequately controlled, despite treatment with at least 2 anti-epileptic medicinal products1
ROI: ONTOZRY®▼ is indicated for the adjunctive treatment of focal-onset seizures with or without secondary generalisation in adult patients with epilepsy who have not been adequately controlled despite a history of treatment with at least 2 anti-epileptic medicinal products2

Drug resistant epilepsy is defined as epilepsy that has not been controlled by 2 antiseizure medicines3
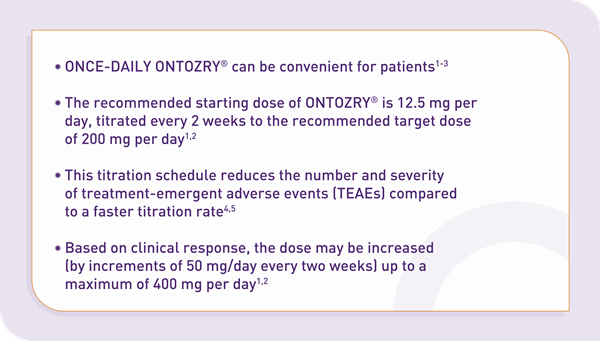
Why choose ONTOZRY®?
Significant unmet need remains for patients with focal-onset epilepsy, particularly for those with uncontrolled focal-onset epilepsy6

In clinical studies of patients with uncontrolled focal seizures, adjunctive ONTOZRY® significantly reduced seizure frequency compared with placebo8
-
- 56% of patients achieved a ≥50% reduction in seizure frequency with ONTOZRY® 200 mg/day vs 25% of patients with placebo when added to SOC8*,†
Seizure freedom is possible with adjunctive ONTOZRY® for some drug-resistant patients8
-
- 21% of patients achieved seizure freedom with ONTOZRY® 400 mg/day vs 1% of patients with placebo when added to SOC.8*,‡ The target dose for ONTOZRY® is 200 mg1,2
ONTOZRY® is generally well tolerated, with a dose-dependent TEAE profile1,2,8
-
- Most common adverse reactions were somnolence, dizziness, fatigue and headache1,2

Footnotes
*In the randomly assigned population at baseline, median number of previous ASMs taken at any time before the start of the study was 3 (range 2 - 4); these might or might not have been ongoing during the study. 74% (n=322/437) of patients were taking 2–3 concomitant ASMs during the study.8
†During the 12-week maintenance phase of fixed-dose, double-blind treatment, responder rates from baseline were 25% (n=26/102) for the placebo group compared with 56% (n=55/98; OR 3.74 [2.06-6.80], 95% CI; p<0.0001) for the ONTOZRY® 200 mg/day dose group.8
‡Seizure freedom was defined as 100% reduction in seizure frequency. During the 12-week maintenance phase, seizure freedom responder rates from baseline were 1% (n=1/102) for the placebo group, 4% (n=4/102) for the ONTOZRY® 100 mg/day group, 11% (n=11/98; p=0.0022) for the ONTOZRY® 200 mg/day group and 21% (n=20/95; p<0.0001) for the ONTOZRY® 400 mg/day group.8
ASM = anti-seizure medication; CI = confidence interval; OR = odds ratio, SOC = standard of care; TEAE = treatment-emergent adverse event.
© NICE 2024 Cenobamate for treating focal onset seizures in epilepsy. Technology appraisal guidance TA753. Available from https://www.nice.org.uk/guidance/ta753. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
- Great Britain: ONTOZRY® Summary of Product Characteristics.
- ROI: ONTOZRY® EMA Summary of Product Characteristics.
- NICE Technology appraisal guidance (TA753). Cenobamate for treating focal onset seizures in epilepsy. 15 December 2021. Available at: https://www.nice.org.uk/guidance/ta753 (last accessed November 2024).
- Sperling MR, et al. Epilepsia. 2020;61(6):1099-1108.
- Steinhoff BJ, et al. Acta Neurol Scand. 2022;146(3):265-75.
- Ioannou P, et al. Brain Behav. 2022;12(9):e2589.
- Chen Z, et al. JAMA Neurol. 2018;75(3):279-86.
- Krauss GL, et al. Lancet Neurol. 2020;19(1):38‒48.
MAT-UKI-0063-P | November 2024

 HarmoniaMentis
HarmoniaMentis