ONTOZRY®▼ (cenobamate) for refractory focal onset epilepsy

ONTOZRY®▼ (cenobamate) for refractory focal onset epilepsy
This promotional website is managed and funded by Angelini Pharma and is intended for UK and Ireland healthcare professionals only.
If you are a patient please click here
What is ONTOZRY ® (cenobamate)?
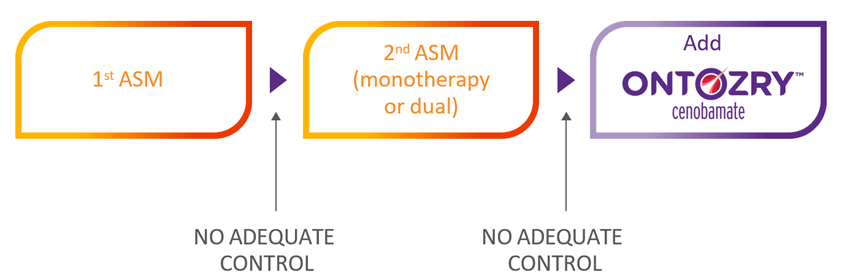
ONTOZRY® (cenobamate) is a medication used for the adjunctive treatment of focal-onset seizures with or without secondary generalisation in adult patients with drug-resistant epilepsy that has not been adequately controlled despite treatment with at least two anti-epileptic medicinal products.1,2 ONTOZRY® gained approval in the UK and the EU.1,3
Why choose ONTOZRY® (cenobamate)?
An unmet need remains for patients with focal-onset epilepsy, particularly for those with uncontrolled focal-onset epilepsy: Approximately 40% of patients with epilepsy experience drug-resistant epilepsy despite treatment advances.4
Drug-resistant epilepsy can have a big impact on the quality of life of patients . Epilepsy can be accompanied by depression and anxiety, and the stigma of epilepsy and health concerns also adds to the burden of patients.4,5 Since seizure freedom is linked to an improved quality of life, achieving better seizure control is a crucial goal in mitigating these negative impacts.4
Clinical studies have shown the efficacy of ONTOZRY®. The drug can reduce seizure frequency in most patients and can help increase the chances of seizure freedom in those with focal-onset epilepsy.6
ONTOZRY® is taken orally once daily. Tablets can be swallowed whole or crushed and dispersed in water for oral or nasogastric administration.1 The once daily administration is convenient for patients and could increase adherence
Learn more about the dosing and administration of ONTOZRY®.
ONTOZRY ®(cenobamate) is thought to have dual action against seizure propagation
The efficacy of ONTOZRY® was shown in clinical studies.6
The Phase IIb, multicentre, randomised, double-blind, placebo-controlled C017 study demonstrated that in patients with uncontrolled focal seizures, adjunctive ONTOZRY® significantly reduced seizure frequency compared with placebo:8
- 21% of patients achieved seizure freedom with ONTOZRY® 400 mg*/day compared to 1% of patients with placebo when used as an adjunctive to current therapies.
- 56% of patients achieved a ≥50% reduction in seizure frequency with ONTOZRY® 200 mg/day vs 25% of patients with placebo when added to standard of care (SOC).
ONTOZRY ®(cenobamate) is thought to have dual action against seizure propagation
ONTOZRY® targets seizure propagation through a dual mechanism of action: it inhibits sodium receptor signalling and increases GABA receptor response. The exact mechanisms by which ONTOZRY® exercises its therapeutic effects in patients with focal-onset seizures is not fully established.1
ONTOZRY ® (cenobamate) is generally well tolerated
ONTOZRY® is generally well tolerated, with a dose-dependent treatment-emergent adverse events (TEAE) profile.9,10
Overall, the safety and tolerability profile of ONTOZRY ® is comparable with other adjunctive ASMs in adults with focal-onset seizures.11
ONTOZRY ®(cenobamate) approval and NICE recommendation
ONTOZRY® is approved for use in the NHS, and it is accepted for restricted use in Scotland by the SMC.7,12
NICE recommends ONTOZRY® as an adjuvant treatment for adults with focal-onset seizures that are not controlled after two or more ASMs.7
Find an overview of current clinical guidelines for the use of ONTOZRY®.
Resources on demand
Explore our on-demand resources, where you can discover comprehensive information about ONTOZRY® and find out what experts think about ONTOZRY®.

Footnotes
*the target dose of ONTOZRY® is 200mg/day.1
Abbreviations
ASM, anti-seizure medications; SOC, standard of care; TEAE, treatment-emergent adverse events.
© NICE 2024 Cenobamate for treating focal onset seizures in epilepsy. Technology appraisal guidance TA753. Available from https://www.nice.org.uk/guidance/ta753. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
- EMA. ONTOZRY® EU Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/ontozry-epar-product-information_en.pdf (last accessed December 2025)
- EMC. ONTOZRY® Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/13012/smpc (last accessed December 2025)
- MHRA. Cenobamate PLGB 53287/0001 -0007. Available at: https://mhraproducts4853.blob.core.windows.net/docs/db54659cb6b0179c6c4fcf6a9e1bad4e85210840 (last accessed December 2025)
- Ioannou P, Foster DL, Sander JW, et al. The burden of epilepsy and unmet need in people with focal seizures. Brain Behav. 2022;12(9):e2589. doi:10.1002/brb3.2589
- Guery D, Rheims S. Clinical management of drug resistant epilepsy: A review on current strategies. Neuropsychiatr Dis Treat. 2021;17:2229-2242. doi:10.2147/NDT.S256699
- Steinhoff BJ, Rosenfeld WE, Serratosa JM, et al. Practical guidance for the management of adults receiving adjunctive cenobamate for the treatment of focal epilepsy-expert opinion. Epilepsy Behav. 2021;123(108270):108270. doi:10.1016/j.yebeh.2021.108270
- NICE. Cenobamate for treating focal onset seizures in epilepsy. Available at: https://www.nice.org.uk/guidance/ta753 (last accessed December 2025)
- Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38-48. doi:10.1016/S1474-4422(19)30399-0
- Steinhoff BJ, Ben-Menachem E, Brandt C, et al. Onset of efficacy and adverse events during Cenobamate titration period. Acta Neurol Scand. 2022;146(3):265-275. doi:10.1111/ane.13659
- Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia. 2020;61(6):1099-1108. doi:10.1111/epi.16525
- Mulheron S, Leahy TP, McStravick M, Doran R, Delanty N. A comparison of cenobamate with other newer antiseizure medications for adjunctive treatment of focal-onset seizures: A systematic review and network meta-analysis. Seizure. 2024;118:80-90. doi:10.1016/j.seizure.2024.04.004
- SMC. Cenobamate (Ontozry). Available at: https://scottishmedicines.org.uk/medicines-advice/cenobamate-ontozry-full-smc2408/ (last accessed December 2025)
MAT-UKI-0282-P | December 2025

 HarmoniaMentis
HarmoniaMentis